Azaheterocycles Based on a,ß-Unsaturated Carbonyls
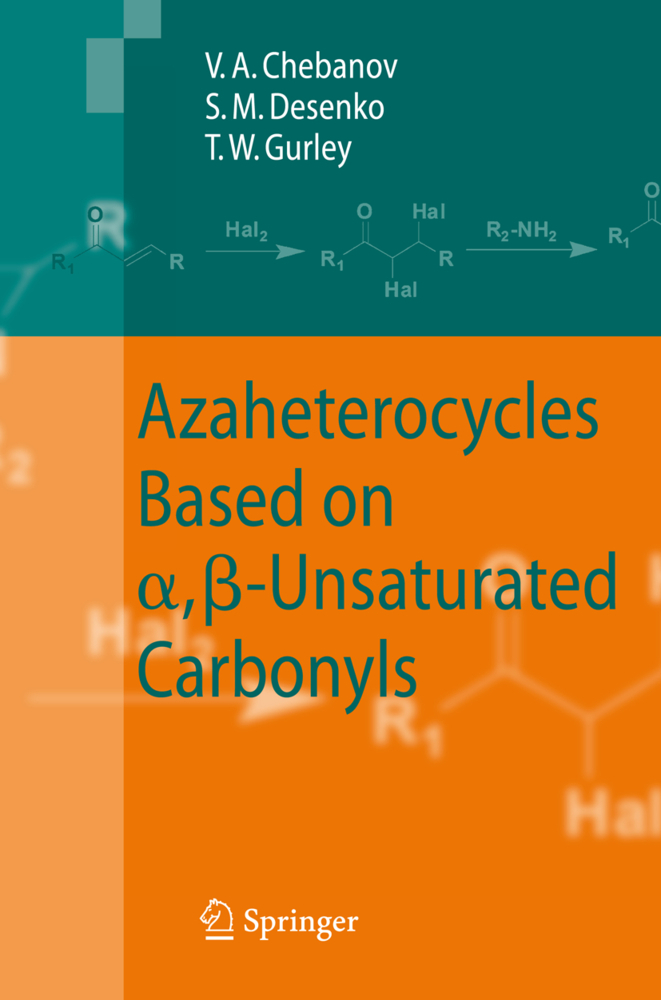
This book is devoted to heterocyclizations of aliphatic and aromatic , -unsaturated carbonyls with various binucleophiles leading to three-, five-, six and seven-membered partially hydrogenated nitrogen-containing heterocycles. During the last decade interest in these classes of organic c- pounds has been experiencing a scientific renaissance owing to their significant role in biological processes in living cells and diverse effects on physiological activities. In addition, such compounds are also more prevalent from the vi- point of classical problems of organic chemistry, among them reactivity, chemo- and regioselectivity, tautomerism, conformational analysis and features of their electronic structure. The character of these problems in the case of partially hydrogenated heterocycles differs sufficiently from that for hetero- omatized and perhydrogenated heterocyclic compounds and investigations in this field very often lead to interesting and unusual results. Extensively characterized cyclocondensations of , -unsaturated carbonyls, their synthetic equivalents and their precursors are the most widespread, facile and generally valid pathway to dihydroazaheterocycles. The popularity and significance of this synthetic approach is based on the high reactivity and availability of unsaturated carbonyl compounds and the precise selectivity of the heterocyclization reactions in comparison with that involving -dicarbonyls. The recent development of combinatorial high-throughput methods and the use of new energy sources such as microwaves and ultrasound to enhance reactions have also increased interest in , -unsaturated carbonyls and their reactions.
Six-Membered Azaheterocycles Based on 1,3-Binucleophiles
Cyclocondensations of 1,2-Diamines.
Three-Membered Azaheterocycles
Five-Membered AzaheterocyclesSix-Membered Azaheterocycles Based on 1,3-Binucleophiles
Cyclocondensations of 1,2-Diamines.
Chebanov, Valentin A.
Desenko, Sergey M.
Gurley, Thomas W.
| ISBN | 978-3-540-68361-2 |
|---|---|
| Artikelnummer | 9783540683612 |
| Medientyp | Buch |
| Copyrightjahr | 2008 |
| Verlag | Springer, Berlin |
| Umfang | VII, 210 Seiten |
| Abbildungen | VII, 210 p. |
| Sprache | Englisch |